|
 |
- Search
| J Korean Med Assoc > Volume 54(9); 2011 > Article |
Abstract
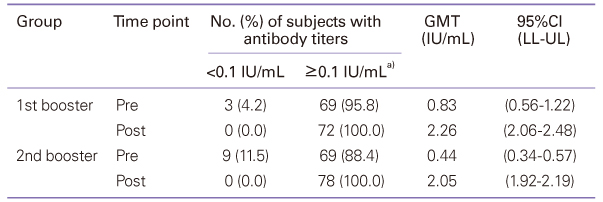
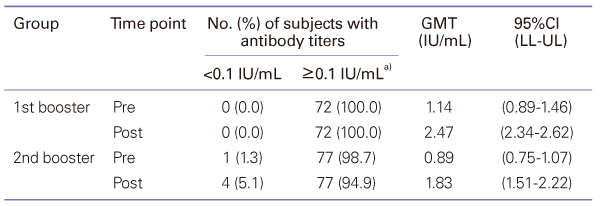
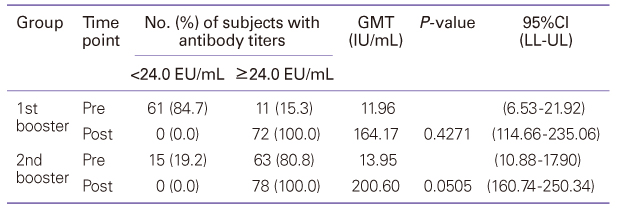
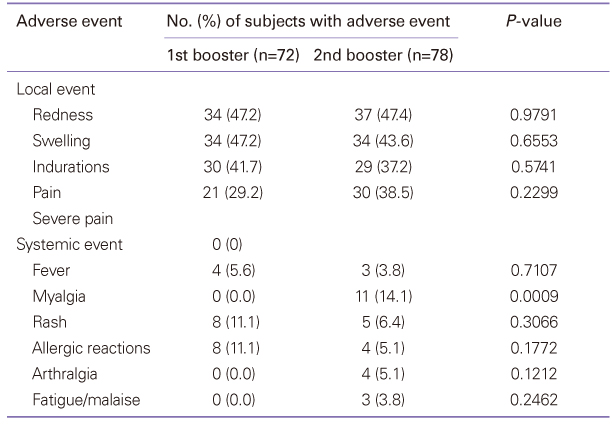
Diphtheria-tetanus-acellular pertussis (DTaP) vaccination must currently be administered three times starting at 2 months of age, at intervals of two months, with the first and second boosters administered at 15 to 8 months and 4 to 6 years of age. A high rate of vaccination is maintained, but studies of the efficiency and safety of booster vaccination are lacking. This study evaluated the immunogenicity and safety of the DTaP booster vaccine. Seventy-two infants who had been vaccinated with the first booster and 78 children who had been vaccinated with the second booster were enrolled in this study. Local and systemic adverse reactions after vaccination were recorded. Sera obtained before and 1 month after booster vaccination were analyzed for antibodies to diphtheria and tetanus toxoid, and anti-pertussis toxin. Diphtheria: The GMT was increased. Tetanus: The geometric mean antibody titer (GMT) was increased. Pertussis: The GMT was increased by 13.72 times and 14.37 times after the first and the second additional vaccination, respectively. Although the seroconversion rate was low prior to the first booster, the average amount of anti-pertussis toxin antibodies before the first additional vaccination was 143.37 EU/mL, which rose to 261.88 EU/mL after the vaccination. The seroconversion rate also increased to 100%. Adverse reactions showed spontaneous resolution within a few days after vaccination. After the second additional vaccination, there was a statistically significant increase in the manifestation of myalgia compared to after the first additional vaccination. In conclusion, DTaP booster vaccination was effective in Korean children, demonstrating that modifications to the current regimen would be unnecessary.
References
1. Decker MD, Edwards KM, Steinhoff MC, Rennels MB, Pichichero ME, Englund JA, Anderson EL, Deloria MA, Reed GF. Comparison of 13 acellular pertussis vaccines: adverse reactions. Pediatrics 1995;96(3 Pt 2):557-566.
2. Edwards KM, Meade BD, Decker MD, Reed GF, Rennels MB, Steinhoff MC, Anderson EL, Englund JA, Pichichero ME, Deloria MA. Comparison of 13 acellular pertussis vaccines: overview and serologic response. Pediatrics 1995;96(3 Pt 2):548-557.
3. Miller E. Overview of recent clinical trials of acellular pertussis vaccines. Biologicals 1999;27:79-86.
4. Salmaso S, Mastrantonio P, Tozzi AE, Stefanelli P, Anemona A, Ciofi degli Atti ML, Giammanco A. Stage III Working Group. Sustained efficacy during the first 6 years of life of 3-component acellular pertussis vaccines administered in infancy: the Italian experience. Pediatrics 2001;108:E81.
5. Liese JG, Meschievitz CK, Harzer E, Froeschle J, Hosbach P, Hoppe JE, Porter F, Stojanov S, Niinivaara K, Walker AM, Beloh-radsky BH. Efficacy of a two-component acellular pertussis vaccine in infants. Pediatr Infect Dis J 1997;16:1038-1044.
6. Schmitt HJ, von Konig CH, Neiss A, Bogaerts H, Bock HL, Schulte-Wissermann H, Gahr M, Schult R, Folkens JU, Rauh W, Clemens R. Efficacy of acellular pertussis vaccine in early childhood after household exposure. JAMA 1996;275:37-41.
7. Simondon F, Preziosi MP, Yam A, Kane CT, Chabirand L, Iteman I, Sanden G, Mboup S, Hoffenbach A, Knudsen K, Guiso N, Wassilak S, Cadoz M. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine 1997;15:1606-1612.
8. Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi AE, Anemona A, Ciofidegli Atti ML, Giammanco A, Panei P, Blackwelder WC, Klein DL, Wassilak SG. Progetto Pertosse Working Group. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N Engl J Med 1996;334:341-348.
9. Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med 1996;334:349-355.
10. Olin P, Rasmussen F, Gustafsson L, Hallander HO, Heijbel H. Ad Hoc Group for the Study of Pertussis Vaccines. Randomised controlled trial of two-component, three-component, and five-component acellular pertussis vaccines com-pared with whole-cell pertussis vaccine. Lancet 1997;350:1569-1577.
11. Sato Y, Sato H. Acellular pertussis vaccine as a solution to the prevention of whooping cough. Curr Opin Infect Dis 1992;5:399-405.
12. Noble GR, Bernier RH, Esber EC, Hardegree MC, Hinman AR, Klein D, Saah AJ. Acellular and whole-cell pertussis vaccines in Japan. Report of a visit by US scientists. JAMA 1987;257:1351-1356.
13. Edwards KM, Decker MD. Acellular pertussis vaccines for infants. N Engl J Med 1996;334:391-392.
14. The Korean Pediatric Society. In: Lee HJ, editor. Diphtheria, pertussis, tetanus vaccines. Immunization guideline 2008;6th ed. Seoul: The Korean Pediatric Society. 75-90.
15. Swartz TA, Saliou P, Catznelson E, Blondeau C, Gil I, Peled T, Havkin O, Fletcher M. Immune response to a diphtheria and tetanus toxoid administration in a three-dose diphtheria tetanus whole-cell pertussis/enhanced inactivated poliovirus vaccination schedule: a 7-year follow up. Eur J Epidemiol 2003;18:827-833.
16. Pichichero ME, Deloria MA, Rennels MB, Anderson EL, Edwards KM, Decker MD, Englund JA, Steinhoff MC, Deforest A, Meade BD. A safety and immunogenicity comparison of 12 acellular pertussis vaccines and one whole-cell pertussis vaccine given as a fourth dose in 15- to 20-month-old children. Pediatrics 1997;100:772-788.
17. Pichichero ME, Green JL, Francis AB, Marsocci SM, Murphy AM, Buscarino C. Antibody response and reactions to completion of a four-dose series with a two-or three-component acellular pertussis vaccine compared to whole cell pertussis vaccine. Scand J Infect Dis 1996;28:159-163.
18. Liese JG, Stojanov S, Zink TH, Froeschle J, Klepadlo R, Kronwitter A, Harzer E, Jow S, Belohradsky BH. Munich Vaccine Study Group. Safety and immunogenicity of Biken acellular pertussis vaccine in combination with diphtheria and tetanus toxoid as a fifth dose at four to six years of age. Pediatr Infect Dis J 2001;20:981-988.
19. Pichichero ME, Edwards KM, Anderson EL, Rennels MB, Englund JA, Yerg DE, Blackwelder WC, Jansen DL, Meade BD. Safety and immunogenicity of six acellular pertussis vaccines and one whole-cell pertussis vaccine given as a fifth dose in four- to six-year-old children. Pediatrics 2000;105:e11.
20. Baraff LJ, Cherry JD, Cody CL, Marcy SM, Manclark CR. DTP vaccine reactions: effect of prior reactions on rate of subsequent reactions. Dev Biol Stand 1985;61:423-428.
21. Cody CL, Baraff LJ, Cherry JD, Marcy SM, Manclark CR. Nature and rates of adverse reactions associated with DTP and DT immunizations in infants and children. Pediatrics 1981;68:650-660.
22. Kang JH, Kim JH, Lee JH, Lee SY, Hong YJ, Kim CH. The immunogenicity and safety of three-component DTaP vaccine in Korean infants. Korean J Pediatr 2007;50:355-362.
23. Rennels MB, Deloria MA, Pichichero ME, Losonsky GA, Englund JA, Meade BD, Anderson EL, Steinhoff MC, Edwards KM. Extensive swelling after booster doses of acellular pertussis-tetanus-diphtheria vaccines. Pediatrics 2000;105:e12.
- TOOLS