1. Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest 2006;116:1167-1173.
2. Pollack AE, Balkin M, Edouard L, Cutts F, Broutet N. WHO/UNFPA Working Group on Sexual and Reproductive Health and HPV Vaccines. Ensuring access to HPV vaccines through integrated services: a reproductive health perspective. Bull World Health Organ 2007;85:57-63.
3. Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices(ACIP). MMWR Recomm Rep 2007;56(RR-2):1-24.
4. American Academy of Pediatrics Committee on Infectious Diseases. Recommended immunization schedules for children and adolescents-United States, 2007. Pediatrics 2007;119:207-208.
5. Saslow D, Castle PE, Cox JT, Davey DD, Einstein MH, Ferris DG, Goldie SJ, Harper DM, Kinney W, Moscicki AB, Noller KL, Wheeler CM, Ades T, Andrews KS, Doroshenk MK, Kahn KG, Schmidt C, Shafey O, Smith RA, Partridge EE, Garcia F. Gynecologic Cancer Advisory Group. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin 2007;57:7-28.
6. Kim KH, Kim JH, Park SE, Shin SH, Oh SH, Lee HJ, Jo DS, Choi EH, Hur JK, Hong YJ. The Committee on Infectious Diseases, The Korean Pediatric Society. Human Papillomavirus Vaccine. Korean J Pediatr 2007;50:811-819.
7. Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998;338:423-428.
8. Moscicki AB, Shiboski S, Broering J, Powell K, Clayton L, Jay N, Darragh TM, Brescia R, Kanowitz S, Miller SB, Stone J, Hanson E, Palefsky J. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998;132:277-284.
9. Ho GY, Burk RD, Klein S, Kadish AS, Chang CJ, Palan P, Basu J, Tachezy R, Lewis R, Romney S. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst 1995;87:1365-1371.
10. Schiffman M, Kjaer SK. Natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr 2003;31:14-19.
11. de Villiers EM, Fauquet C, Broker TR, Bernard HU, Zur HH. Classification of papillomaviruses. Virology 2004;324:17-27.
12. Bonnez W, Reichman RC. In: Mandell GL, Bennet JE, Dolin R, editor. Papillomaviruses. Principles and practice of Infectious Disease 2005;6th ed. Elsevior Inc.. 1841-1855.
13. Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003;348:518-527.
14. Clifford G, Franceschi S, Diaz M, Muñoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 2006;24:S3. 26-34.
15. Duensing S, Munger K. Mechanisms of genomic instability in human cancer: insights from studies with human papillomavirus oncoproteins. Int J Cancer 2004;109:157-162.
16. Stanley M. Immune responses to human papillomavirus. Vaccine 2006;24:S16-S22.
17. Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis 2000;181:1911-1919.
18. Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003;157:218-226.
19. Manhart LE, Holmes KK, Koutsky LA, Wood TR, Kenney DL, Feng Q, Kiviat NB. Human papillomavirus infection among sexually active young women in the United States: implications for developing a vaccination strategy. Sex Transm Dis 2006;33:502-508.
20. Watts DH, Koutsky LA, Holmes KK, Goldman D, Kuypers J, Kiviat NB, Galloway DA. Low risk of perinatal transmission of human papillomavirus: results from a prospective cohort study. Am J Obstet Gynecol 1998;178:365-373.
21. Moscicki AB, Schiffman M, Kjaer S, Villa LL. Updating the natural history of HPV and anogenital cancer. Vaccine 2006;24:S42-S51.
22. Castellsague X, Munoz N. Cofactors in human papillomavirus carcinogenesis-role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr 2003;31:20-28.
23. Ministry of Health and Welfare. 2002 Annual Report of the Korea Central Cancer Registry (Published in 2003).
24. Korea National Statistical Office. Annual Report on the Cause of Death Statistics (published in 2005).
25. Shin HR, Lee DH, Herrero R, Smith JS, Vaccarella S, Hong SH, Jung KY, Kim HH, Park UD, Cha HS, Park S, Touze A, Muñoz N, Snijders PJ, Meijer CJ, Coursaget P, Franceschi S. Prevalence of human papillomavirus infection in women in Busan, South Korea. Int J Cancer 2003;103:413-421.
26. Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, Kong HJ, Rha SH, Jung SI, Kim JI, Jung KY, van Doorn LJ, Quint W. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis 2004;190:468-476.
27. Shin HR, Seoh K, Song KJ, Park EC, Choi GS, Lee HY, Oh JK, Kim BK, Kim JY. A report of investigation of disease burden associated with human papillomavirus infection in Korea Korean Center for Disease Control and Prevention. (published in 2007).
28. Stanley M, Lowy DR, Frazer I. Prophylactic vaccines: underlying mechanisms. Vaccine 2006;24:S106-S113.
29. Kahn JA. Vaccination as a prevention strategy for human papillomavirus-related diseases. J Adolesc Health 2005;37:S6. S10-S16.
30. Noad R, Roy P. Virus-like particles as immunogens. Trends Microbiol 2003;11:438-444.
31. Kim YT. Prophylactic vaccine for cervical carcinoma. J Korean Med Assoc 2007;50:151-158.
32. Ahn WS. Recent concepts for human papillomavirus vaccine. J Korean Med Assoc 2007;50:778-784.
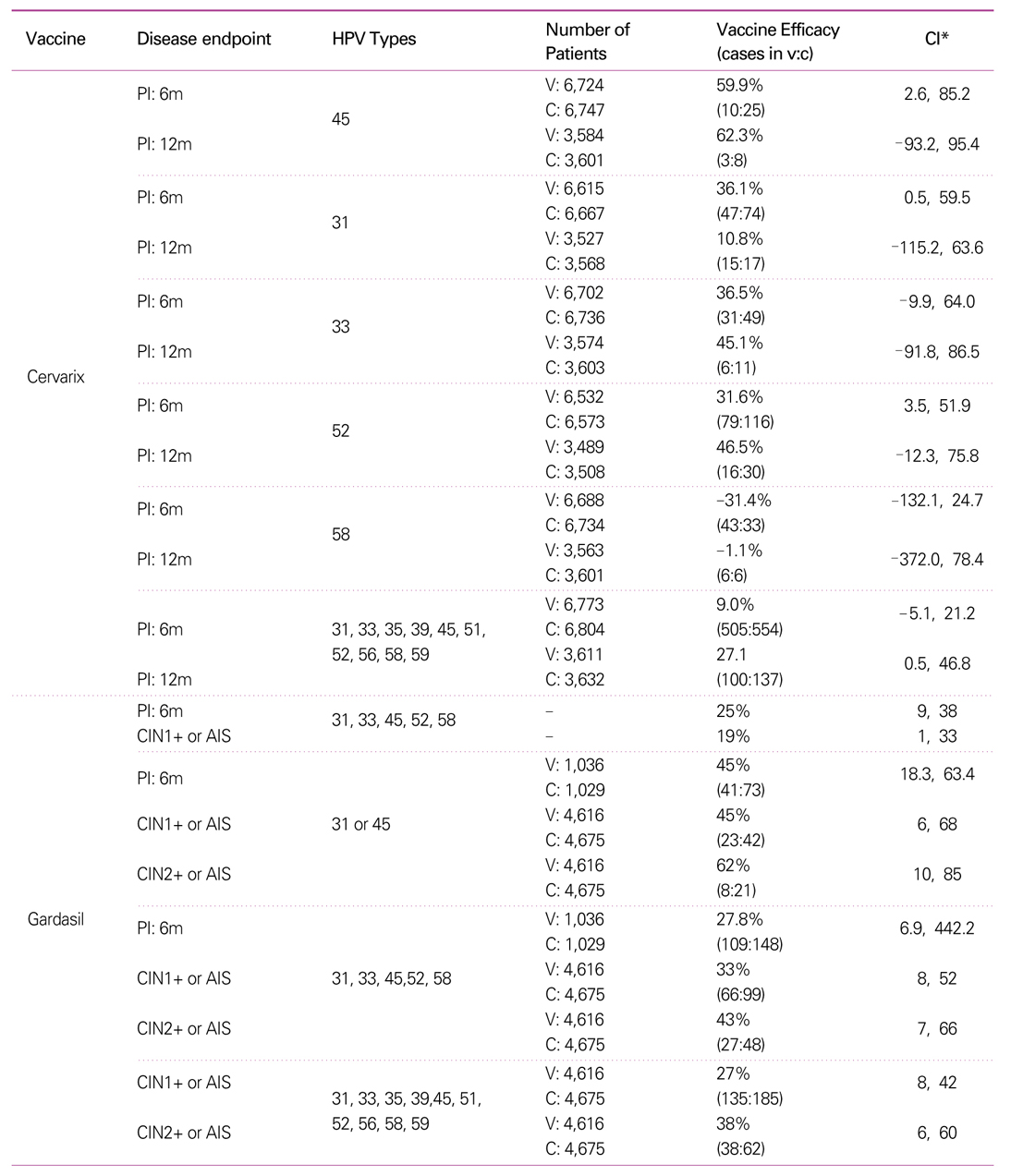
33. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, de Carvalho NS, Skinner SR, Harper DM, Hedrick JA, Jaisamrarn U, Limson GA, Dionne M, Quint W, Spiessens B, Peeters P, Struyf F, Wieting SL, Lehtinen MO, Dubin G. HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccineagainst infection with human papillomavirus types 16 and 18 in young women: aninterim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007;369:2161-2170.
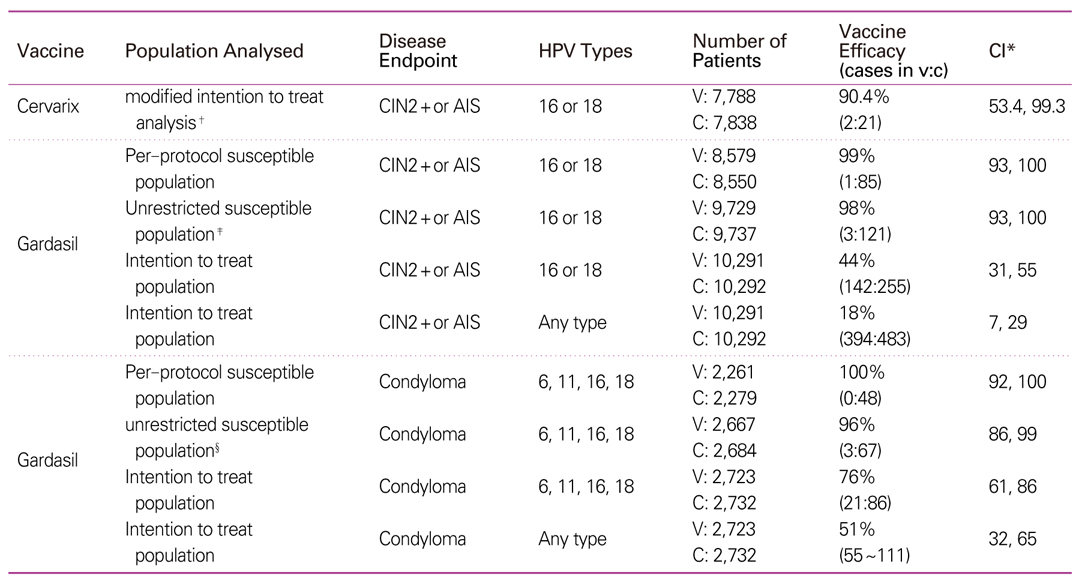
34. Ault KA. Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 2007;369:1861-1868.
35. Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J, Taddeo FJ, Railkar R, Esser MT, Sings HL, Nelson M, Boslego J, Sattler C, Barr E, Koutsky LA. Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007;356:1928-1943.
36. Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, Olsson SE, Høye J, Steinwall M, Riis-Johannessen G, Andersson-Ellstrom A, Elfgren K, KroghG , Lehtinen M, Malm C, Tamms GM, Giacoletti K, Lupinacci L, Railkar R, Taddeo FJ, Bryan J, Esser MT, Sings HL, Saah AJ, Barr E. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006;95:1459-1466.
37. Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, Iversen OE, Høye J, Steinwall M, Riis-Johannessen G, Andersson-Ellstrom A, Elfgren K, von Krogh G, Lehtinen M, Paavonen J, Tamms GM, Giacoletti K, Lupinacci L, Esser MT, Vuocolo SC, Saah AJ, Barr E. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 2007;25:4931-4939.
38. Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G. HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bilvalent L1virus-like particle vaccine against human papillomavirus types 16 and 18: follow up from a randomised control trial. Lancet 2006;367:1247-1255.
39. Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, Esser MT. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol 2003;10:108-115.
40. Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green T, Brown M, Smith J, Chirmule N, Barr E, Jansen KU, Esser MT. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol 2005;12:959-969.
41. Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, Roteli-Martins CM, Teixeira J, Blatter MM, Korn AP, Quint W, Dubin G. GlaxoSmithKline HPV Vaccine Study Group. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004;364:1757-1765.
42. Reisinger KS, Block SL, Lazcano-Ponce E, Samakoses R, Esser MT, Erick J, Puchalski D, Giacoletti KE, Sings HL, Lukac S, Alvarez FB, Barr E. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007;26:201-209.
43. Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, Castellsagué X, Rusche SA, Lukac S, Bryan JT, Cavanaugh PF Jr, Reisinger KS. Protocol 016 Study Group. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics 2006;118:2135-2145.
44. Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, Brown DR, Ferenczy A, Harper DM, Koutsky LA, Kurman RJ, Lehtinen M, Malm C, Olsson SE, Ronnett BM, Skjeldestad FE, Steinwall M, Stoler MH, Wheeler CM, Taddeo FJ, Yu J, Lupinacci L, Railkar R, Marchese R, Esser MT, Bryan J, Jansen KU, Sings HL, TammsGM , Saah AJ, Barr E. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 2006;24:5571-5583.
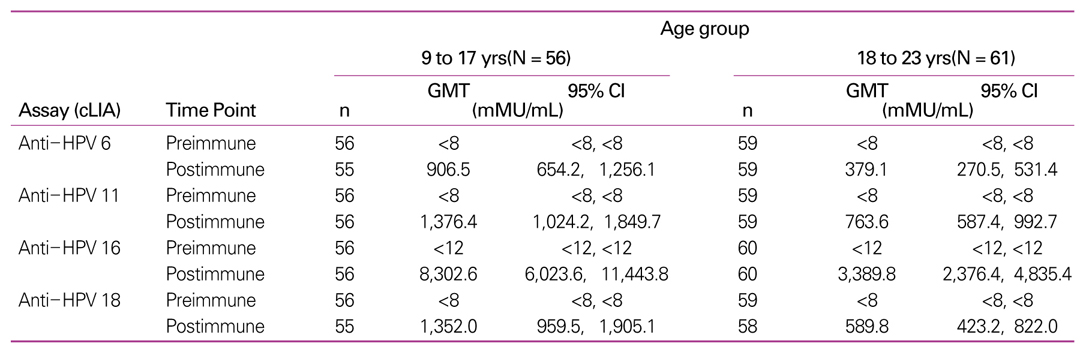
45. Kang S, Kim KH, Kim YT, Kim YT, Kim JH, Song YS, Shin SH, Ryu HS, Han JW, Kang JH, Park SY. Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: a randomized, placebo-controlled trial in 176 Korean subjects. Int J Gynecol Cancer 2007.
46. Munoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006;24:S3. 1-10.
47. Brown D. FUTURE Study Group. HPV Type 6/11/16/18 Vaccine: First analysis of cross-protection against persistent infection, cervical intraepithelial neoplasia (CIN), and adenocarcinoma in situ (AIS) caused by oncogenic HPV types in addition to 16/18 2007;09. Chicago: ICAAC. September 2007.
48. Product approval information-licensing action [package insert]. Gardasil (quadrivalent human papillomavirus types 6, 11, 16, 18). Food and Drug Administration Whitehouse Station, NJ: Merck & Co.. Available at
http://www.fda.gov/cber/label/HPVmer060806LB.pdf
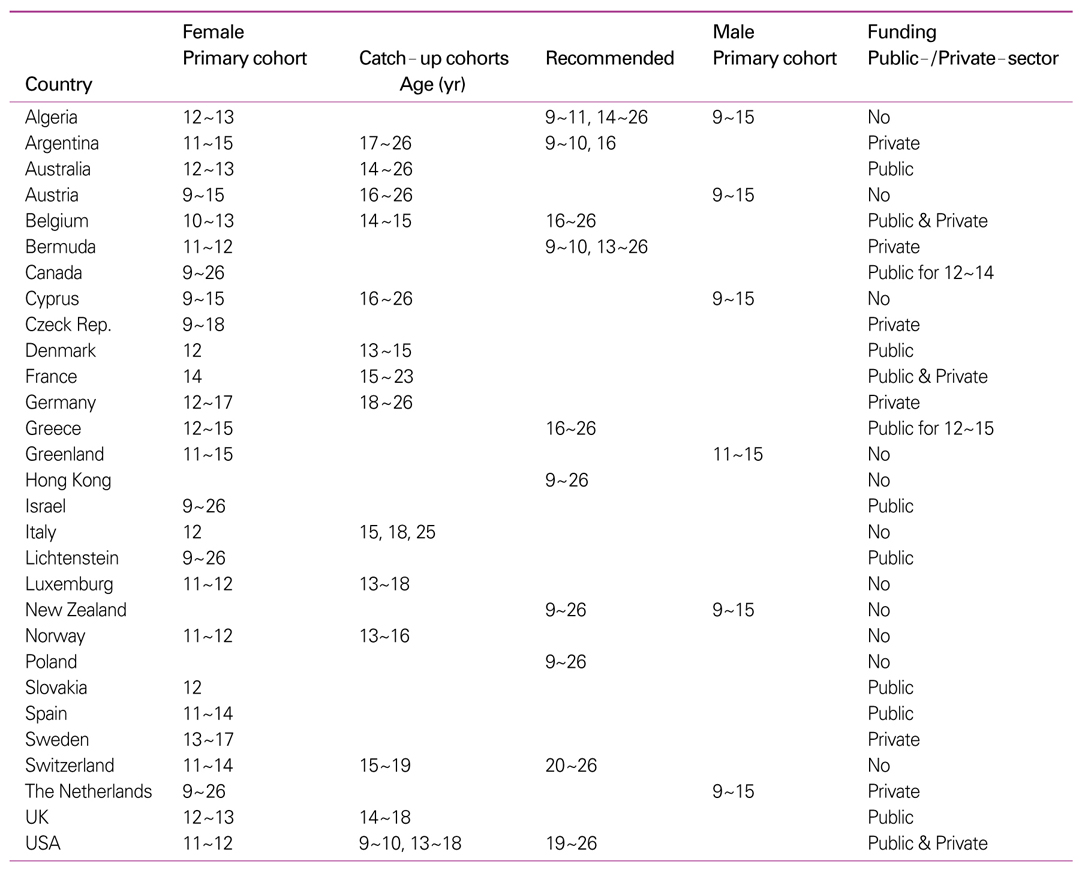
50. Soldan K, Dillner J. Comparable strategies needed to evaluate human papillomavirus vaccine efficiency across Europe. Euro Surveill 2006;11:E061123.3.
51. Kudjawu Y, Lévy-Bruhl D, Celentano LP, O'Flanagan D, Salmaso S, Lopalco P, Mullins N, Bacci S. VENICE working group. The current status of HPV and rotavirus vaccines in national immunisation schedules in the EU-preliminary results of a VENICE survey. Euro Surveill 2007;12:E070426.1.
52. Howell-Jones R. Human papillomavirus vaccination: the United Kingdom's recommendation and update on European licensure and efficacy data. Euro Surveill 2007;12:E071115.4.