|
 |
- Search
| J Korean Med Assoc > Volume 48(9); 2005 > Article |
Abstract
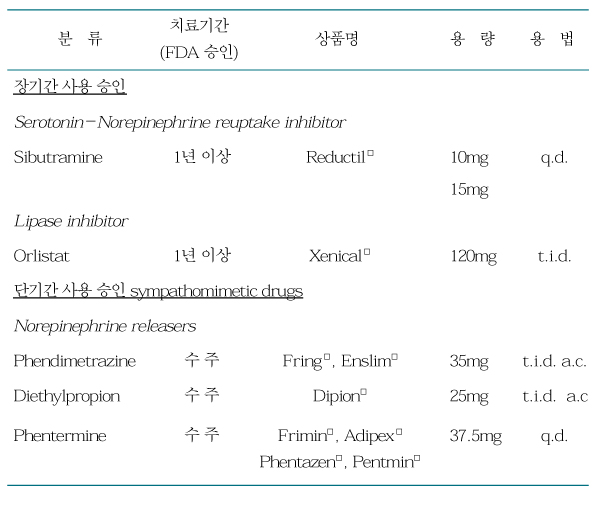
Obesity results from an imbalance between energy intake and energy expenditure. Drugs can shift this balance in a favorable way by reducing food intake, altering metabolism, and by increasing energy expenditure. All patients with obesity should make efforts to change their lifestyle behaviors to decrease energy intake and increase physical activity. Lifestyle modifications also should be a component of all other levels of therapy. Pharmacotherapy can be a useful adjunctive measure for well-selected patients. Anti-obesity pharmacological treatment is indicated when the patient's body mass index (BMI) is >25 kg/m2 or when the patient's BMI is >23 kg/m2 with co-morbidities such as diabetes, hypertension, hypercholesterolemia, and coronary artery disease. Obesity is a chronic disease that requires long-term therapy for successful long-term weight management. Often, the patients regain their lost weight after therapy is discontinued, so it should be stressed that obesity is not a curable disease, and thus the maintenance of the reduced weight is very important. Obese patients must be examined in detail regarding mood, obesity-related complications or conditions, current medications, eating habits, and history of drug side-effects. The physicians' choice for anti-obesity medications is based on both the patient's medical conditions and long-term safety and efficacy of antiobesity drugs.
References
1. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. Xenical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155-161.
2. Hutton B, Fergusson D. Changes in body weight and serum lipid profile in obese patients treated with orlistat in addition to a hypocaloric diet: a systematic review of randomized clinical trials. Am J Clin Nutr 2004;80:1461-1468.
3. Hansen DL, Astrup A, Toubro S, Finer N, Kopelman P, Hilsted J, et al. Predictors of weight loss and maintenance during 2 years of treatment by sibutramine in obesity. Results from the European multi-centre STORM trial. International Journal of Obesity 2004;28:378-383.
4. One-Year Outcome of a Combination of Weight Loss Therapies for Subjects With Type 2 Diabetes, A randomized trial. Diabetes Care 2003;26:2505-2511.
5. Kim SH, Lee YM, Jee SH, Nam CM. Effect of sibutramine on weight loss and blood pressure: a meta-analysis of controlled trials. Obes Res 2003;11:1116-1123.
6. Bray GA, Ryan DH. In: Bray GA, Bouchard C, editor. Sympathomimetic and serotoninergic drugs used to treat obesity. Handbook of obesity, Clinical applications 2004;2nd ed. Marcel Dekker, Inc.: USA. 201-238.
7. Wilding J, Van Gaal L, Rissanen A, Vercruysse F, Fitchet M. OBES-002 Study Group. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. International Journal of Obesity 2004;28:1399-1410.
8. Anderson JW, Greenway FL, Fujioka K, Gadde KM, Mc-Kenney J, O'Neil PM. Bupropion SR enhances weight loss: a 48-week double-blind, placebo- controlled trial. Obes Res 2002;10:633-641.
9. Gadde KM, Franciscy DM, Wagner HR 2nd, Krishnan KR. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA 2003;289:1820-1825.
10. Goldstein DJ, Rampey AH Jr, Enas GG, Potvin JH, Fludzinski LA, Levine LR. Fluoxetine: a randomized clinical trial in the treatment of obesity. Int J Obes Relat Metab Disord 1994;18:129-135.
11. Norris S, Zhang X, Avenell A, Gregg E, Schmid Ch, Lau J. Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;25:CD004096.
12. Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. RIO-Europe Study Group. Effects of the cannabi-oid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from RIO-Europe study. Lancet 2005;365:1389-1389.
- TOOLS
-
METRICS

-
- 1 Crossref
- Scopus
- 1,011 View
- 1 Download
-
Related articles in
J Korean Med Assoc -
For Better Continuing Medical Education2004 March;47(3)