 |
 |
- Search
| J Korean Med Assoc > Volume 54(6); 2011 > Article |
Abstract
Lower urinary tract symptoms (LUTS) are classified into three groups: storage, voiding, and post-micturition symptoms. The most popular causes of LUTS are benign prostatic hyperplasia (BPH) and overactive bladder (OAB). Although BPH is a pathologic term, clinically, we use this when patients have LUTS due to benign prostatic enlargement and obstruction. OAB is defined as urgency, with or without urge incontinence, usually with frequency and nocturia. Currently α1-adrenoceptor antagonists are the most common drug treatment for BPH, and are thought to act by relaxing the prostatic smooth muscle. They are all effective for the treatment of LUTS/BPH. 5α-reductase inhibitors, such as fiansteride and dutasteride, are another treatment option for BPH symptoms, which reduce the prostatic volume by inducing epithelial atrophy. Long-term combination therapy with alpha-1-blockers and 5α-reductase inhibitors reduces the risk of the overall clinical progression of BPH significantly more than does treatment with either drug alone. Antimuscarinics are the mainstay for the treatment of OAB. Antimuscarinics competitively block muscarinic receptors of all subtypes but with variations in selectivity for the different subtypes. When they are used for the treatment of OAB, they are active during the storage phase of the bladder, with little or no effect on voiding contractions. Desmopressin acetate is a synthetic analogue of Arginin vasopressin, which has been proven effective for the treatment of nocturnal polyuria in LUTS.
References
1. Lepor H, Auerbach S, Puras-Baez A, Narayan P, Soloway M, Lowe F, Moon T, Leifer G, Madsen P. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol 1992;148:1467-1474.
2. Boyle P, Robertson C, Manski R, Padley RJ, Roehrborn CG. Meta-analysis of randomized trials of terazosin in the treat-ment of benign prostatic hyperplasia. Urology 2001;58:717-722.
3. Lepor H, Knapp-Maloney G, Sunshine H. A dose titration study evaluating terazosin, a selective, once-a-day alpha 1-blocker for the treatment of symptomatic benign prostatic hyperplasia. J Urol 1990;144:1393-1397.
4. MacDonald R, Wilt TJ, Howe RW. Doxazosin for treating lower urinary tract symptoms compatible with benign prostatic obstruction: a systematic review of efficacy and adverse effects. BJU Int 2004;94:1263-1270.
5. Roehrborn CG. Efficacy and safety of once-daily alfuzosin in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a randomized, placebo-controlled trial. Urology 2001;58:953-959.
6. Van Kerrebroec P, Jardin A, van Cangh P, Laval KU. ALFORTI Study Group. Long-term safety and efficacy of a once-daily formulation of alfuzosin 10 mg in patients with symptomatic benign prostatic hyperplasia: open-label extension study. Eur Urol 2002;41:54-60.
7. Nickel JC, Elhilali M, Emberton M, Vallancien G. Alf-One Study Group. The beneficial effect of alfuzosin 10 mg once daily in 'real-life' practice on lower urinary tract symptoms (LUTS), quality of life and sexual dysfunction in men with LUTS and painful ejaculation. BJU Int 2006;97:1242-1246.
8. Schulman CC, Cortvriend J, Jonas U, Lock TM, Vaage S, Speakman MJ. European Tamsulosin Study Group. Tamsulosin, the first prostate-selective alpha 1A-adrenoceptor antagonist. Analysis of a multinational, multicentre, open-label study assessing the long-term efficacy and safety in patients with benign prostatic obstruction (symptomatic BPH). Eur Urol 1996;29:145-154.
9. Chapple CR, Montorsi F, Tammela TL, Wirth M, Koldewijn E, Fernandez Fernandez E. European Silodosin Study Group. Silodosin therapy for lower urinary tract symptoms in men with suspected benign prostatic hyperplasia: results of an international, randomized, double-blind, placebo- and active-controlled clinical trial performed in Europe. Eur Urol 2011;59:342-352.
10. Kawabe K, Yoshida M, Homma Y. Silodosin Clinical Study Group. Silodosin, a new alpha1A-adrenoceptor-selective antagonist for treating benign prostatic hyperplasia: results of a phase III randomized, placebo-controlled, double-blind study in Japanese men. BJU Int 2006;98:1019-1024.
11. Ikemoto I, Kiyota H, Ohishi Y, Abe K, Goto H, Kishimoto K, Miki K. Usefulness of tamsulosin hydrochloride and naftopidil in patients with urinary disturbances caused by benign prostatic hyperplasia: a comparative, randomized, two-drug crossover study. Int J Urol 2003;10:587-594.
12. Gotoh M, Kamihira O, Kinukawa T, Ono Y, Ohshima S, Origasa H. Tokai Urological Clinical Trial Group. Comparison of tamsulosin and naftopidil for efficacy and safety in the treatment of benign prostatic hyperplasia: a randomized controlled trial. BJU Int 2005;96:581-586.
13. McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, Albertsen P, Roehrborn CG, Nickel JC, Wang DZ, Taylor AM, Waldstreicher J. Finasteride Long-Term Efficacy and Safety Study Group. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med 1998;338:557-563.
14. Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, Fowler IL, Rittmaster RS. REDUCE Study Group. Effect of dutasteride on the risk of prostate cancer. N Engl J Med 2010;362:1192-1202.
15. McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, Lepor H, McVary KT, Nyberg LM Jr, Clarke HS, Crawford ED, Diokno A, Foley JP, Foster HE, Jacobs SC, Kaplan SA, Kreder KJ, Lieber MM, Lucia MS, Miller GJ, Menon M, Milam DF, Ramsdell JW, Schenkman NS, Slawin KM, Smith JA. Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003;349:2387-2398.
16. Diokno AC, Appell RA, Sand PK, Dmochowski RR, Gburek BM, Klimberg IW, Kell SH. OPERA Study Group. Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc 2003;78:687-695.
17. Van Kerrebroeck P, Kreder K, Jonas U, Zinner N, Wein A. Tol-terodine Study Group. Tolterodine once-daily: superior efficacy and tolerability in the treatment of the overactive bladder. Urology 2001;57:414-421.
18. Chapple CR, Martinez-Garcia R, Selvaggi L, Toozs-Hobson P, Warnack W, Drogendijk T, Wright DM, Bolodeoku J. STAR study group. A comparison of the efficacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: results of the STAR trial. Eur Urol 2005;48:464-470.
19. Haab F, Cardozo L, Chapple C, Ridder AM. Solifenacin Study Group. Long-term open-label solifenacin treatment associated with persistence with therapy in patients with overactive bladder syndrome. Eur Urol 2005;47:376-384.
20. Nitti VW, Dmochowski R, Sand PK, Forst HT, Haag-Molkenteller C, Massow U, Wang J, Brodsky M, Bavendam T. Efficacy, safety and tolerability of fesoterodine for overactive bladder syndrome. J Urol 2007;178:2488-2494.
21. Chapple C, Van Kerrebroeck P, Tubaro A, Haag-Molkenteller C, Forst HT, Massow U, Wang J, Brodsky M. Clinical efficacy, safety, and tolerability of once-daily fesoterodine in subjects with overactive bladder. Eur Urol 2007;52:1204-1212.
22. Herschorn S, Swift S, Guan Z, Carlsson M, Morrow JD, Brodsky M, Gong J. Comparison of fesoterodine and tolterodine extended release for the treatment of overactive bladder: a head-to-head placebo-controlled trial. BJU Int 2010;105:58-66.
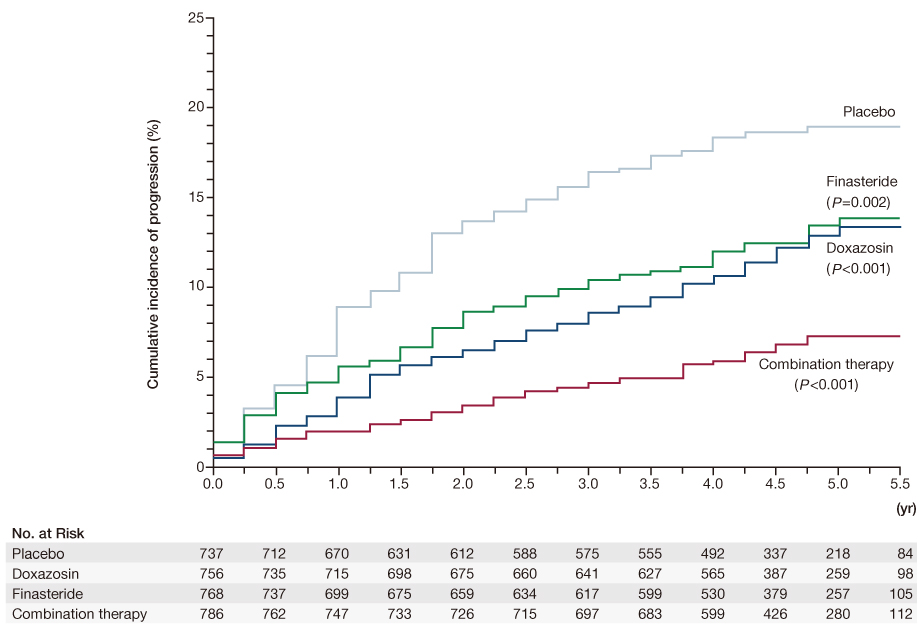
Figure 1
Cumulative incidence of progression of benign prostatic hyperplasia in Medical Therapy of Prostatic Symptoms study. Progression was defined by an increase of at least 4 points over base line in the American Urological Association symptom score, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection. P-values are for the comparison with placebo. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. (From McConnell JD, et al. N Engl J Med 2003; 349: 2387-2398, with permission from Massachusetts Medical Society) [15].
- TOOLS
-
METRICS

-
- 2 Crossref
- Scopus
- 1,299 View
- 14 Download
-
Related articles in
J Korean Med Assoc -
Surgical treatment for rotator cuff tears: the way it ought to be2022 November;65(11)
Diagnosis and treatment of women with recurrent urinary tract infection2022 September;65(9)
Surgical treatment for obesity2022 July;65(7)
Surgical treatment of senile spinal diseases2021 March;64(3)





